Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O
Yoshii, Kenji; Ikeda, Naoshi*; Mori, Shigeo*
no journal, ,
no abstracts in English
Shimojo, Kojiro; Naganawa, Hirochika; Kubota, Fukiko*; Goto, Masahiro*
no journal, ,
no abstracts in English
Nakagawa, Seiko*; Taguchi, Mitsumasa; Kojima, Takuji
no journal, ,
no abstracts in English
Tsukada, Kazuaki; Toyoshima, Atsushi; Haba, Hiromitsu*; Asai, Masato; Akiyama, Kazuhiko*; Ishii, Yasuo; Tome, Hayato*; Nishinaka, Ichiro; Sato, Tetsuya; Ichikawa, Takatoshi; et al.
no journal, ,
no abstracts in English
Toyoshima, Atsushi; Haba, Hiromitsu*; Tsukada, Kazuaki; Asai, Masato; Akiyama, Kazuhiko*; Ishii, Yasuo; Tome, Hayato*; Nishinaka, Ichiro; Sato, Tetsuya; Ichikawa, Takatoshi; et al.
no journal, ,
no abstracts in English
Asano, Masaharu; Chen, J.; Yamaki, Tetsuya; Maekawa, Yasunari; Yoshida, Masaru
no journal, ,
To develop a highly chemically stable proton conducting membrane for use in direct methanol fuel cell (DMFC), doubly crosslinked membranes were successfully prepared by the combination of chemical crosslinking and radiation crosslinking. Four monomers consisting of m,p-methylstyrene, p-tert-butylstyrene, divinylbenzene, and 1,2-bis(p,p-vinylphenyl)ethane were first introduced into poly(ethylene-co-tetrafluoroethylene) (ETFE) films by means of -grafting process. Then, the multiple
-grafting process. Then, the multiple  -crosslinking was performed for the ETFE base film containing grafted-chains and subsequent sulfonation. As a result, this proton conducting membrane showed a very low permeability compared with that of Nafion in high methanol feed concentration.
-crosslinking was performed for the ETFE base film containing grafted-chains and subsequent sulfonation. As a result, this proton conducting membrane showed a very low permeability compared with that of Nafion in high methanol feed concentration.
Septiani, U.; Chen, J.; Asano, Masaharu; Maekawa, Yasunari; Kubota, Hitoshi*; Yoshida, Masaru
no journal, ,
Both the effect of pre-irradiation atmosphere, argon and air, on the radiation grafting of styrene into poly(ethylene-co-tetrafluoroethylene) (ETFE) films and the durability of the relevant ETFE-based polymer electrolyte membranes (PEM) were investigated. It was found that the ETFE-based PEM prepared under air showed a very low durability compared with that under argon. This is due to the introduced unstable ether bond between the grafted chain and backbone film.
Chen, J.; Asano, Masaharu; Maekawa, Yasunari; Yoshida, Masaru
no journal, ,
The object of this study is to improve the durability of radiation-grafted polymer electrolyte membranes (PEM), which were prepared by post-grafting p-methyl styrene with chemical crosslinkers such as divinylbenzene (DVB), 1,2-bis(p,p-vinylphenyl)ethane (BVPE), and triallyl cyanurate (TAC) into ETFE films after  -preirradiation and subsequent sulfonation. Among the three different chemical crosslinkers, DVB showed the most pronounced efficiency for durability of the PEM obtained, in close relation to introduction of tightly crosslinked network structures based on DVB.
-preirradiation and subsequent sulfonation. Among the three different chemical crosslinkers, DVB showed the most pronounced efficiency for durability of the PEM obtained, in close relation to introduction of tightly crosslinked network structures based on DVB.
Miyamoto, Nobuyoshi; Yamaguchi, Daisuke; Nakato, Teruyuki*; Koizumi, Satoshi; Hashimoto, Takeji
no journal, ,
The structure of liquid crystalline sols of niobate nanosheets was observed by USANS, SANS, and USAXS as the function of the average lateral size L and the volume fraction 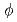 of the nanosheets. The nanosheets sols were obtained by a reaction of K
of the nanosheets. The nanosheets sols were obtained by a reaction of K Nb
Nb O
O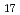 crystals with propylammonium followed by washing with water. The sol was ultrasonicated for 10-180 min and diluted to yield the series of the samples with different L and
crystals with propylammonium followed by washing with water. The sol was ultrasonicated for 10-180 min and diluted to yield the series of the samples with different L and 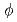 . At q
. At q 0.1, the peaks due to the lamellar structure of the liquid crystal were observed in the scattering curves. The basal spacing was ca. 40 nm, independent of L, at
0.1, the peaks due to the lamellar structure of the liquid crystal were observed in the scattering curves. The basal spacing was ca. 40 nm, independent of L, at 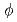 = 3.6 vol % and it decreased with decreasing
= 3.6 vol % and it decreased with decreasing 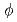 . The scattering curves at 0.01
. The scattering curves at 0.01  q
q  0.1 depended largely on L, or the form factor of the nanosheets. At q
0.1 depended largely on L, or the form factor of the nanosheets. At q  0.01 nm
0.01 nm , the power law of q
, the power law of q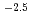 was observed independent of L, indicating the presence of the liquid crystalline domains with mass fractal structure.
was observed independent of L, indicating the presence of the liquid crystalline domains with mass fractal structure.
Wada, Atsushi; Watanabe, Masayuki; Yamanoi, Yoshinori*; Murata, Masaki*; Nishihara, Hiroshi*
no journal, ,
Lanthanide complexes with linear and cyclic octadentate oligopyridine-amine ligands were newly synthesized, and their molecular structures were determined by single-crystal X-ray crystallography. All of the complexes formed with the distorted CSAP geometry, the symmetry of which was higher for the complexes with the cyclic ligand than those with the linear ligand. The Eu and Tb
and Tb complexes showed intense luminescence due to energy transfer from the ligand to the metal center (antenna effect). The Eu
complexes showed intense luminescence due to energy transfer from the ligand to the metal center (antenna effect). The Eu complexes with the linear ligand showed more intense emissions attributed to the 5D0
complexes with the linear ligand showed more intense emissions attributed to the 5D0 7F2 transition than the complex with the cyclic ligand, which can be explained by the decrease in symmetry around the lanthanide ion. The coordination of water molecules to Eu
7F2 transition than the complex with the cyclic ligand, which can be explained by the decrease in symmetry around the lanthanide ion. The coordination of water molecules to Eu and Tb
and Tb ions was strongly inhibited by surrounding the metal ions with the cyclic ligand, resulting in an appearance of intense luminescence in water. These results indicate that the symmetry and stability of lanthanide complexes, and thus the luminescence properties, can be successfully controlled by tuning the geometrical structures of multi-dentate ligands.
ions was strongly inhibited by surrounding the metal ions with the cyclic ligand, resulting in an appearance of intense luminescence in water. These results indicate that the symmetry and stability of lanthanide complexes, and thus the luminescence properties, can be successfully controlled by tuning the geometrical structures of multi-dentate ligands.
Numakura, Masahiko; Ikeda, Atsushi; Kobayashi, Toru; Shiwaku, Hideaki; Suzuki, Shinichi; Yaita, Tsuyoshi
no journal, ,
no abstracts in English